Swamy Yeleswaram
Job Title
VP
Employer
Incyte Corporation

Review details about the recently announced changes to study and work permits that apply to master’s and doctoral degree students. Read more
You're ready to work at the forefront of pharmaceutical sciences advancement. Take your education to the next level with a PhD at UBC Pharm Sci. It's where you'll work shoulder to shoulder with other leading experts in the field of pharmaceutical sciences, contributing knowledge, developing solutions, and shaping the future of health care. Come to work every day at one of the world's most inspiring campuses, where you will find exceptional mentors and supervisors, and state-of-the-art facilities.
At UBC Pharm Sci, our research has shaped our outstanding international reputation. This is the place to collaborate with some of the world's foremost pharmaceutical experts, generating relevant, evidence-based and industry-focused research that makes a positive impact on broader society.
As a PhD student, you will embark upon a journey of academic discovery by working alongside renowned researchers who are at the top of their fields. Right from the onset of the program, you will be welcomed by a vibrant collegial community, receive individualized guidance to shape your customized study plan, and receive mentorship from senior researchers. During the program, you will enrich your knowledge and build your skills set to prepare for careers in academia or industry, while exploring research frontiers in world-class facilities. Our graduates are often highly sought after by the pharmaceutical industry for their expertise in drug discovery and development.
Our PhD program attracts some of the brightest and most curious scientific minds, so you can expect to work alongside some of the best scholars to inspire you. Our student body is diverse both in terms of educational backgrounds and global talent. More than half of our graduate students join us from countries outside of Canada, bringing various perspectives to share. We offer orientation events to help new students integrate into life here in Vancouver, at UBC and within the Faculty. And our Pharmaceutical Sciences Graduate Student Society (PharGS) will help support you through your educational journey and make you feel right at home.
To be considered for admission to the Pharm Sci PhD program, a complete application must be submitted by January 15th. Incomplete applications will not be considered.
For International students: This includes the submission of a copy of the OFFICIAL IELTS or TOEFL transcript (directly from the Testing Agency).
The Faculty of Graduate and Postdoctoral Studies establishes the minimum admission requirements common to all applicants, usually a minimum overall average in the B+ range (76% at UBC). The graduate program that you are applying to may have additional requirements. Please review the specific requirements for applicants with credentials from institutions in:
Each program may set higher academic minimum requirements. Please review the program website carefully to understand the program requirements. Meeting the minimum requirements does not guarantee admission as it is a competitive process.
Applicants from a university outside Canada in which English is not the primary language of instruction must provide results of an English language proficiency examination as part of their application. Tests must have been taken within the last 24 months at the time of submission of your application.
Minimum requirements for the two most common English language proficiency tests to apply to this program are listed below:
Overall score requirement: 100
Reading
22
Writing
22
Speaking
22
Listening
22
Overall score requirement: 7.0
Reading
6.5
Writing
6.5
Speaking
6.5
Listening
6.5
Some programs require additional test scores such as the Graduate Record Examination (GRE) or the Graduate Management Test (GMAT). The requirements for this program are:
The GRE is not required.
All applicants have to submit transcripts from all past post-secondary study. Document submission requirements depend on whether your institution of study is within Canada or outside of Canada.
Many programs require a statement of interest, sometimes called a "statement of intent", "description of research interests" or something similar.
Students in research-based programs usually require a faculty member to function as their thesis supervisor. Please follow the instructions provided by each program whether applicants should contact faculty members.
Permanent Residents of Canada must provide a clear photocopy of both sides of the Permanent Resident card.
All applicants must complete an online application form and pay the application fee to be considered for admission to UBC.
If you're passionate about health sciences research that makes a difference in patients' lives, then UBC Pharmaceutical Sciences is your place. Innovative, collaborative, impactful and widely recognized, our groundbreaking research is relevant to today's problems.
Some examples of our research include work in the following areas:
A PhD in pharmaceutical sciences is the entry point to countless research opportunities in drug discovery and development. Whether it’s genomics and individualized therapy, nanomedicine, chemical biology, pharmacology, or epidemiology, we offer options for you to explore and collaborate. Our research themes include:
Molecular & Systems Pharmacology is comprised of areas such as drug metabolism, pharmacokinetic modeling, cancer biology/pharmacology, diabetes, cardiovascular pharmacology, neuroscience/neuropharmacology, receptor pharmacology, and pharmacogenomics. This highly interdisciplinary theme embodies research directed at the interactions of drugs with therapeutic targets, and covers fundamental questions of the molecular and cellular basis of individual variations in response to drugs, mechanisms of drug action and the pathogenesis of diseases. These studies are used to inform and optimize the development and delivery of drug intervention regimes for clinical practice.
Nanomedicine & Chemical Biology applies our expertise in the chemical biology of the fabrication and handling of nanoscopic materials to drug discovery and delivery. Sensing and screening technologies are also an important focus.
Epidemiology and Health Outcomes covers our activities in epidemiological analysis, health outcomes and health economics research seeking solutions for the predictive enhancement of intervention strategies for practical and preventive healthcare. The impact of this work is used to shape policy to optimize the allocation of health care resources as well as defining the efficacy of healthcare interventions and strategy.
Opened in 2012, the award-winning Pharmaceutical Sciences building is a quarter-million-square-foot, state-of-the-art learning and research facility in the heart of the UBC campus. Functional, striking and always thrumming with activity, the building is home to cutting-edge equipment, laboratories and research spaces that we make available for use by the scientific public. Our building houses modern, modular labs designed specifically for the type of research intended for the space. Among an array of state-of-the-art scientific equipment, the Faculty houses a modern mass spectrometer facility for pharmacokinetic and drug metabolism studies, and a Sequenom Mass-ARRAY system for genetic studies.
| Fees | Canadian Citizen / Permanent Resident / Refugee / Diplomat | International |
|---|---|---|
| Application Fee | $118.50 | $168.25 |
| Tuition * | ||
| Installments per year | 3 | 3 |
| Tuition per installment | $1,912.84 | $3,360.55 |
| Tuition per year (plus annual increase, usually 2%-5%) | $5,738.52 | $10,081.65 |
| Int. Tuition Award (ITA) per year (if eligible) | $3,200.00 (-) | |
| Other Fees and Costs | ||
| Student Fees (yearly) | $1,169.35 (approx.) | |
| Costs of living | Estimate your costs of living with our interactive tool in order to start developing a financial plan for your graduate studies. | |
Applicants to UBC have access to a variety of funding options, including merit-based (i.e. based on your academic performance) and need-based (i.e. based on your financial situation) opportunities.
This results in a net balance (any funding provided to the student minus tuition and fees) mean of $31,686 and median of $29,278.
All applicants are encouraged to review the awards listing to identify potential opportunities to fund their graduate education. The database lists merit-based scholarships and awards and allows for filtering by various criteria, such as domestic vs. international or degree level.
Many professors are able to provide Research Assistantships (GRA) from their research grants to support full-time graduate students studying under their supervision. The duties constitute part of the student's graduate degree requirements. A Graduate Research Assistantship is considered a form of fellowship for a period of graduate study and is therefore not covered by a collective agreement. Stipends vary widely, and are dependent on the field of study and the type of research grant from which the assistantship is being funded.
Graduate programs may have Teaching Assistantships available for registered full-time graduate students. Full teaching assistantships involve 12 hours work per week in preparation, lecturing, or laboratory instruction although many graduate programs offer partial TA appointments at less than 12 hours per week. Teaching assistantship rates are set by collective bargaining between the University and the Teaching Assistants' Union.
Academic Assistantships are employment opportunities to perform work that is relevant to the university or to an individual faculty member, but not to support the student’s graduate research and thesis. Wages are considered regular earnings and when paid monthly, include vacation pay.
Canadian and US applicants may qualify for governmental loans to finance their studies. Please review eligibility and types of loans.
All students may be able to access private sector or bank loans.
UBC has working agreements with MPower Financing - an organization providing international students with no-cosigner, no-collateral education loans to study in Canada - and Windmill Microlending - an organization providing loans to skilled immigrants.
Many foreign governments provide support to their citizens in pursuing education abroad. International applicants should check the various governmental resources in their home country, such as the Department of Education, for available scholarships.
The possibility to pursue work to supplement income may depend on the demands the program has on students. It should be carefully weighed if work leads to prolonged program durations or whether work placements can be meaningfully embedded into a program.
International students enrolled as full-time students with a valid study permit can work on campus for unlimited hours and work off-campus for no more than 24 hours a week during academic sessions.
A good starting point to explore student jobs is the UBC Work Learn program or a Co-Op placement.
Students with taxable income in Canada may be able to claim federal or provincial tax credits.
Canadian residents with RRSP accounts may be able to use the Lifelong Learning Plan (LLP) which allows students to withdraw amounts from their registered retirement savings plan (RRSPs) to finance full-time training or education for themselves or their partner.
Please review Filing taxes in Canada on the student services website for more information.
Applicants have access to the cost estimator to develop a financial plan that takes into account various income sources and expenses.
48 students graduated between 2005 and 2013. Of these, career information was obtained for 42 alumni (based on research conducted between Feb-May 2016):
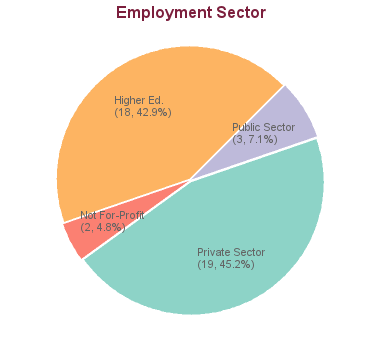
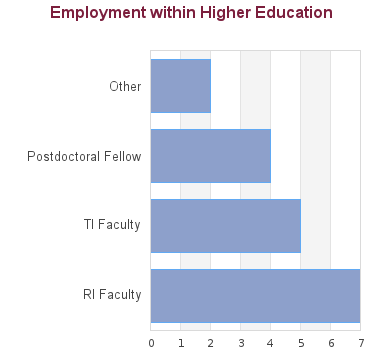
With a tailored PhD in pharmaceutical sciences, the door is open to numerous research career options in drug discovery and development. Our graduates have gone on to create R&D companies in the pharmaceutical and biotechnology sectors, and developed and commercialized therapeutic products in the treatment of various diseases. While a number of our PhD graduates opt for careers in academia or government, the majority of our alumni thrive in industry.
These statistics show data for the Doctor of Philosophy in Pharmaceutical Sciences (PhD). Data are separated for each degree program combination. You may view data for other degree options in the respective program profile.
| 2024 | 2023 | 2022 | 2021 | 2020 | |
|---|---|---|---|---|---|
| Applications | 46 | 76 | 49 | 56 | 56 |
| Offers | 7 | 10 | 9 | 8 | 4 |
| New Registrations | 4 | 7 | 7 | 5 | 4 |
| Total Enrolment | 46 | 44 | 40 | 42 | 36 |
Students in research-based programs usually require a faculty member to function as their thesis supervisor. Please follow the instructions provided by each program whether applicants should contact faculty members.
These videos contain some general advice from faculty across UBC on finding and reaching out to a supervisor. They are not program specific.
This list shows faculty members with full supervisory privileges who are affiliated with this program. It is not a comprehensive list of all potential supervisors as faculty from other programs or faculty members without full supervisory privileges can request approvals to supervise graduate students in this program.
| Year | Citation |
|---|---|
| 2025 | Dr. Thiombane used chemogenomic CRISPR screens to study a class of widely used cancer chemotherapeutic drugs named anthracyclines. Her research provided a global view of all the drug-gene interactions for several clinically used anthracyclines. Her data supports the renewed interest in the clinical use of aclarubicin for cancer chemotherapy. |
| 2025 | In Dr. Oveisi's research, a multi-method approach was used to examine cancer’s impact on adolescent and young adult sexual and reproductive health. Quantitative analysis showed higher risks of early menopause, painful sex, and infertility. Qualitatively, patients discussed impacts on personal, relationship, and community levels, and barriers to receiving appropriate care. |
| 2024 | Dr. Tan developed a 3D human-based lympho-reticular model and a multi-organ-on-a-chip platform to simulate atopic diseases. She further investigated the therapeutic effects of a novel small molecule drug for treating atopic conditions on this platform. |
| 2024 | Dr. Rebić used nationally representative data to generate policy-relevant information on the Canadian burden of medication non-adherence, individual and healthcare system factors increasing the risk for ineffective care, and the impact of sex and gender-related inequities. Her thesis provides real-world evidence for universal Pharmacare in Canada. |
| 2024 | Dr. Lee developed a decision-analytic model for evaluating long-term benefits of early interventions for asthma at the population level. He generated key evidence on the disease course of asthma and demonstrated the utility of this model by showing far-reaching benefits of reducing unnecessary infant antibiotic exposure on the burden of asthma. |
| 2024 | Dr. Vo examined racial disparity in health outcomes and healthcare participation. He found that racialized individuals experience inadequate pain management, and are less likely to participate in health research due to distrust. These findings highlight the impact of structural racism on healthcare access and stress the urgent need for systemic changes to achieve health equity. |
| 2024 | Dr. Rowley studied protein arginine N-methyltransferase 2 (PRMT2), an enzyme found to be dysregulated in disease. He developed new techniques to study PRMT2 and how it interacts with other proteins. Findings from his research suggest that PRMT2 orchestrates the activities of other methyltransferases. |
| 2024 | Dr. Soukhtehzari studied glycobiology of breast cancer and identified two novel polysialylated proteins that contributed in breast cancer progression and studied their function and roles in metastasis and breast cancer patients prognosis. |
| 2024 | Dr. Lee explored heparanase’s role in providing heart energy and its changes in diabetes. He also found that heparanase overexpression led to development of physiological cardiac hypertrophy, however, its actions are negated during diabetes, resulting in heart failure. Further investigation may enhance the management of diabetic heart disease. |
| 2024 | Dr. Chao developed immune-boosting lipid nanoparticles to enhance therapy for peritoneal metastasis. His formulations demonstrated improved immune activation, tumor eradication, and long-term anti-tumor immunity. This research offers significant potential for advancing cancer immunotherapy through nanomedicine. |
Pharmaceutical Sciences covers research areas of nanomedicine, drug delivery; drug metabolism, pharmacokinetics and toxicology; pharmacogenomics and pharmacogenetics; diabetes, cardiovascular and molecular pharmacology; neuropharmacology; cancer pharmacology; pharmaceutical health outcomes and pharmacotherapeutics; and pharmaceutical education.
Departments/Programs may update graduate degree program details through the Faculty & Staff portal. To update contact details for application inquiries, please use this form.
